New Treatments Emerge as Sarcoidosis Yields Up its Secrets
Authors:
22 June 2002 (Revision 2.2: 18 Jan 2003)
Abstract
Background:
Sarcoidosis is a hyper-inflammatory disorder of uncertain etiology. The objective of this study was to identify patients who were reporting hypersensitivities to sunlight, and investigate whether this trait might help to identify the etiology of sarcoid inflammation.
Results:
After studying patients who were able to control their Sarcoidosis symptoms simply by limiting their exposure to sunlight,
and others who were benefiting from Angiotensin Receptor Blockade, we
identified, and confirmed, a working hypothesis detailing
each step of the molecular chemistry leading to the inflammation seen in Sarcoidosis.
The study also identified, and validated, a number of novel treatments.
Discussion:
We found that the secosteroid hormone 1,25-dihydroxyvitamin D (1,25-D) clearly plays an important part in the etiology and symptomology of Sarcoidosis, regardless of the calcemic state of the patient. We described an inflammatory biochemistry which identified 1,25-D and Angiotensin II as the key hormonal mediators.
Many patients’ symptoms were eased when the concentration of the metabolite 25-hydroxyvitamin D (25-D) was reduced by isolation from sunlight, and the removal of all sources of Vitamin D from the diet. 25-D provides fuel for the unregulated extra-renal production of the secosteroid. This novel therapy also improved markers of disease activity, such as Angiotensin Converting Enzyme, and Alkaline Phosphatase, at the same time that the 1,25-D levels were being normalized.
The D-Ratio, serum 1,25-D (pg/ml) divided by 25-D (ng/ml), gives an indication of the amount of nonrenal 1,25-D being produced within the granulomatous inflammation. It can be used to monitor a patient’s response to therapy.
Angiotensin II plays a vital role in the inflammatory cycle, and a novel form of Angiotensin Receptor Blockade was confirmed as a probable therapeutic alternative to the use of systemic corticosteroids.
The etiology of Sarcoidosis has remained a mystery since its discovery over a century ago. It is a hyper-inflammatory disease primarily treated with systemic corticosteroids. Many patients achieve remission during steroid treatment. Those who do not remit, face the prospect of a lifetime of steroid usage, with the possibility of concomitant severe side effects, including Osteoporosis, Avascular Necrosis, and Diabetes.
Recent studies [1,2,3] have identified that Sarcoidosis most probably has a bacterial pathogenesis. We build on that knowledge by bringing together a number of previously uncoordinated in-vitro studies [4,5,6,7,8,9,10,11,12,13,14] to form a coherent, verifiable, working description of the inflammatory proliferation in sarcoidosis.
Paracrine vs. Endocrine biochemistries
Many centers are studying sarcoid inflammation at the paracrine level, examining the cytokine and chemokine biochemistry.
Our interest, however, is primarily in the endocrine system, particularly in the roles of the hormones that have an anomalous
presentation in sarcoid patients.
1,25-dihydroxyvitamin D and Sunlight
The secosteroid hormone 1,25-dihydroxyvitamin D (1,25-D) is the active vitamin D metabolite, which can be directly produced by the action of sunlight on the skin [23]. Sunlight also causes the skin to produce Vitamin D and 25-hydroxyvitamin D (25-D), which are both stored in the body’s fat reserves. This storage ensures that an adequate concentration of the active hormone (1,25-D) will be available, even in the absence of sunlight. The stored Vitamin D is hydroxylated to 25-D in the liver, and this 25-D is then converted into the active hormone within the kidneys, which maintain 1,25-D homeostasis.
Adams [15] noted that the sarcoid "disease-activated macrophage" also hydroxylates 25-D to 1,25-D, and that the hormone produced in the sarcoid inflammation was in excess of the body’s requirements. He suggested that this excess hormone might be reduced by "controlling vitamin D intake and sunlight exposure in susceptible hosts".
However, we suspected that the atypical regulation of this hormone in sarcoid patients [4,15,16,17] might not only be a result of the "disease-activated macrophage", but that the excess 1,25-D might actually be a cause of the run-away inflammatory process leading to the production of sarcoid granuloma.
Cadranel [9] had come to a similar conclusion in 1995, proposing that 1,25-D was responsible for the "formulation and maturation of granulomas", but was unable to identify the remaining element(s) of the inflammatory cycle.
We set out to identify patients who were convinced that exposure to sunlight exacerbated their sarcoid symptoms. We hoped that they might be able to provide the clue which would allow us to define the precise role of 1,25-D in sarcoid inflammation.
1,25-dihydroxyvitamin D in
Sarcoidosis
Before the advent of oral corticosteroids, large ‘therapeutic doses’ of Vitamin D were often administered to Sarcoidosis patients. Anderson, et al [16], found that although normal adults could tolerate 150,000 IU of exogenous Vitamin D, some sarcoid patients exhibited toxicity after only 9,000 IU. Scadding [17] reported that some of his patients could not tolerate any exogenous Vitamin D at all, and that those patients who were most sensitive to Vitamin D were less likely to achieve remission.
This enhanced sensitivity of Sarcoidosis patients to Vitamin D makes Adams’ suggestion of lowering 1,25-D concentrations by "controlling .. sunlight exposure" very difficult to implement. Almost total isolation from sunlight is required before many sarcoid patients begin to significantly depress the circulating levels of 1,25-D, and very few patients have found this to be socially acceptable.
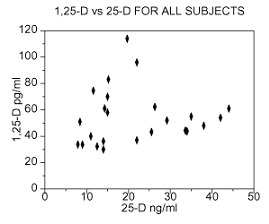
Figure 1 is a scatter plot of all serum 1,25-D and serum 25-D values measured in our study cohort of sarcoidosis patients (21 patients, 24 data sets)

The mean value of all 1,25-D data from the cohort is 54.8, with a 90% confidence interval from 47.4 to 62.1.
The Danish population study [18] identified a mean value of 29 pg/ml for the serum 1,25-D measured in a "normal" population, with a 9.5 Standard Deviation (σ). This is consistent with Merck [24], which suggests using 45 pg/ml as the upper limit of normal for serum 1,25-D.
Whether we accept Merck’s recommendation or the Danish population study, it is clear that the mean value of 1,25-D taken from our cohort of sarcoid patients is elevated by comparison with a normal population. The T test of hypothesis "that the cohort mean is greater than the Danish population mean", 29 pg/ml, is accepted with greater than 95% probability.
In fact, the hypothesis "that the cohort mean value is greater than 45 pg/ml", Merck’s upper limit of normal, is also accepted at the 95% probability level.
Yet the great majority of these patients have never been diagnosed with hypercalcemia. Their elevated level of serum 1,25-D clearly indicates that this secosteroid hormone itself plays a key part in the etiology, and the symptomology, of Sarcoidosis, independent of any linkage with the calcium metabolism.
We have found that sarcoid patients typically present with elevated levels of 1,25-D. With the exception of those receiving D supplementation for osteoporosis, the 25-D level is usually lower than the normal mean (μ < 25 ng/ml [18] P=0.8). This is consistent with Adams’ observation [15] that sarcoid inflammation is responsible for the energetic conversion of 25-D to 1,25-D within the granuloma.
We have consequently developed a metric we call the ‘D-Ratio’, 1,25-D (pg/ml) divided by 25-D (ng/ml), which has been extremely helpful in evaluating patients, and tracking the progress of therapies. Its ‘normal’ mean is 1.25 (σ=0.5), but sarcoid patients usually present with much higher values. We believe it reflects Adams’ energetic extra-renal production of 1,25-D by the inflammatory biochemistry.
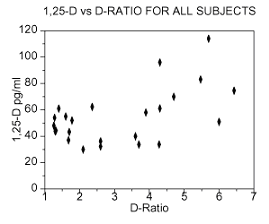
Figure 2 is a scatter plot of our study cohort, with serum 1,25-D plotted against the corresponding D-Ratio.
Now the results logically divide into two regions, those patients with D-Ratios less than 3 and those with D-Ratios greater than 3.
For the region where the D-Ratio < 3, the 1,25-D values, although elevated, tend not to be extreme, while in the right hand region (D-Ratio >3), where especially energetic conversion of 25-D to 1,25-D is occurring in the granuloma, we see that extreme values of 1,25-D are the norm, rather than the exception.
The Symptoms of Fatigue, Asthenia, Pain and
Headache
A recent study of sarcoid patients in the Netherlands [19] found that fatigue was the symptom reported most frequently (71%). Other reported symptoms included asthenia (25.5%), muscle pain (37.3%), headache (27.1%), and tension (30.9%). The study did not show a significant reduction in any of these symptoms subsequent to administration of corticosteroids.
These are all symptoms which can result from Hypervitaminosis D, an excessive concentration of 1,25-D in the bloodstream. The patients in our study were suffering from a sampling of these symptoms.
As our study had already identified elevated levels of 1,25-D, the diagnosis of Hypervitaminosis D was routine. After reducing their intake of dietary Vitamin D, and sunlight, most of these patients reported significant symptomatic relief.
Both the absolute level of 1,25-D and the D-ratio should be assessed in any sarcoid patient presenting with fatigue, asthenia, muscle pain, headache or tension.
Three unimpeachable reports of significant relief after isolation from
sunlight
We identified three unimpeachable patients with biopsy proven sarcoidosis, all of whom were prednisone-free. Two had been diagnosed in the 1970’s, the third more recently. All have controlled their sarcoid symptoms by isolation of both their skin from sunlight and their eyes from bright lights. Isolation had been variously practiced for periods of 15, 2 and 1 years.
The symptoms that all these patients attribute to be a consequence of transient exposure to the sun include fatigue, paresthesia, neuropathy, and asthenia.
Despite their markedly different environmental conditions, all three patients report that even their neuropathy and pain can be relieved by isolation, and the symptoms recur for several days after exposure to sunlight. Yet none of these patients have ever exhibited clinical hypercalcemia or hypercalciuria.
Skin lesions, neuropathy and pain exacerbated by sunlight in
volunteer
A recent study [20] demonstrated that ‘therapeutic doses’ of Vitamin D (100,000 IU) remain in the body for several months after administration. Given that previous in-vitro studies [4-13] had clearly established that 1,25-D contributes to the pathology of granulomatous inflammation, we judged it unethical to risk the health of patients, already in symptomatic remission, by directly challenging them with Vitamin D.
However, one of these patients, entirely of her own volition, decided to prove to herself that exposure to sunlight had in fact been the cause of her suffering.
Symptomatic since 1998, with conclusive diagnosis by biopsy in March, 2001, this patient had subsequently achieved symptomatic remission by staying indoors and avoiding dietary Vitamin D. She had no discernible skin lesions.
But after 6 weeks of trying to resume a ‘normal lifestyle’, she was feeling very sick indeed, with a serum ACE of 99 IU/L, a D-Ratio of 4.3, and a 1,25-D of 61.1 pg/ml (range 20-45 [24]). Her Alkaline Phosphatase had risen to a high 185 IU/L (range 25-150), but Calcium was still ‘normal’ at 10.0 mg/dL. A cough had returned, along with cognitive problems, neuropathy, insomnia and pain in the chest, ankles and hip. Sarcoid skin lesions had broken out on her neck, back, and buttocks.
She decided she just could not take any more suffering, and began to make sure she stayed indoors during the daylight hours. 7 weeks later her ACE had fallen to 88, her D-Ratio to 3.7, her 1,25-D to 34 and her Calcium to 9.4 Skin lesions on her neck and back had disappeared. The lesions on her buttocks, however, had not receded sufficiently, and it was decided to commence Minocycline, 100mg q.o.d. She was on no other medication.
In another 4 weeks her ACE was 58, her D-Ratio was 2.6, and her 1,25-D was 32.3. Alkaline Phosphatase had dropped to 109, Calcium to 9.3 The skin lesions on her buttocks were still discernible, receding at a satisfactory pace.
Of particular interest is the change in her serum ACE. It is normal for 1,25-D to exhibit a negative endocrine modulation of the renin-angiotensin system [21], yet this patient clearly exhibited a direct (positive) correlation between her 1,25-D and serum ACE as she was exposed to sunlight (which is presumed to primarily mediate systemic 25-D and 1,25-D concentrations).
1,25-dihydroxyvitamin D’s role in Sarcoid
Inflammation
The role of 1,25-D in the etiology of sarcoid granuloma has been thoroughly investigated in-vitro. It has been found to enhance the production of monocytes from hematopoetic stem cells [5], to enhance the differentiation of those monocytes into macrophages and multi-nucleated giant cells [6], and, through its interaction with T-lymphocytes, to play an important part in the regulation of granulomatous reactions within bronchoalveolar lavage from sarcoidosis patients [7].
Cadranel [9] had also noted this key role of 1,25-D in the "formulation and maturation of granulomas", but was unable to identify any "paracrine mediator" which could catalyze the run-away proliferation of the inflammation.
Angiotensin Receptor Blockade provides the
key
However, two of the “unimpeachable” patients, along with several from the standard cohort, have been variously using the Angiotensin Receptor Blockers (ARBs) Avapro, Diovan and Benicar, to lower their sensitivity to solar exposure [22]. Whenever the ‘sunlight symptoms’ reappear, an increased dosage of ARB can regain symptomatic control. The ARB itself is not known to have any direct effect on 1,25-D homeostasis. The ARB can only be acting on this hormone indirectly, by blockading Angiotensin II’s pro-inflammatory activity in the sarcoid macrophages.
The anomalous reaction of these Sarcoidosis patients to Angiotensin Blockade, and the anomalous positive endocrine modulation of the renin-angiotensin system (noted above), have allowed us to identify Angiotensin II as the ‘paracrine mediator’, and thus to delineate each stage of the sarcoid inflammatory biochemistry.
3. "The Angiotensin Hypothesis"
We have concluded that sunlight fuels the inflammation of sarcoidosis, via 1,25-D and Angiotensin II, in the following manner:
As the circulating concentration of 1,25-D increases within the inflamed tissue, a much larger quantity of hematopoetic stem cells differentiate to produce monocytes [5]. Monocyte differentiation into macrophages and epithelioid giant cells is enhanced [6]. The differentiating macrophages and giant cells release Angiotensin Converting Enzyme [10]. This ACE catalyzes Angiotensin I to form Angiotensin II [10] (A-II). The A-II then binds at A-II Type 1 receptors on the macrophages and activated T-lymphocytes [7,8,10,11], stimulating Nuclear Factor-kappaB (NF-κB) to signal the release for a cascade of Th1 cytokines [12]. At least one of these cytokines, Gamma Interferon, increases the amount of 25-D being converted to 1,25-D in the macrophages [13], which in turn catalyses the differentiation of monocytes into even more macrophages and giant cells.
Normally this inflammatory cycle is self-limiting, but, in the case of sarcoid patients, 1,25-D levels are poorly controlled, leading to upregulated production of monocytes, and their upregulated differentiation into the macrophages and epithelioid giant cells characteristic of sarcoid granuloma.
Reichel, et al [4], demonstrated that lipopolysaccharide from gram-negative bacteria stimulated the generation of 1,25-D within pulmonary alveolar macrophages from sarcoid patients. A bacterial pathogenesis [1,2,3] is therefore consistent with the initial increase in paracrine 1,25-D concentrations needed to trigger the run-away inflammatory biochemistry described above.
Testing the hypothesis
The blockade of A-II Type 1 receptors has been shown (in-vitro) to reduce production of the Th1 cytokines [12], including
TNF-α [25], an action which would interdict this inflammatory process.
Angiotensin Receptor Blockers are currently indicated for hypertension. There is thus a simple clinical test of this hypothesis available for patients who are suffering both from Sarcoidosis and mild hypertension.
We have found that Benicar (Olmesartan Medoxomil), administered as 40mg every 6 to 8 hours, provides a very effective angiotensin blockade.
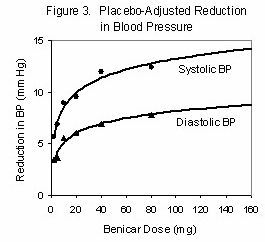 There are two issues in the selection and dosage of the ARB. As you can see from the pressor effect vs. dosage for Benicar (Figure 1),
about 90% of the ultimate pressor effect can be achieved with only 40mg, once per day. But this dose is not well tolerated by sarcoid patients. Partly this may be the result of the additional Angiotensin receptors in the inflamed tissues [8], all of which have to be blocked, and partly it may be due to an increased production of serum ACE by macrophages in response to the partial blockade. Sarcoid patients experience symptoms ranging from increased fatigue to psychedelic dreams [22] when prescribed ARBs just once daily, the customary prescription for hypertension
There are two issues in the selection and dosage of the ARB. As you can see from the pressor effect vs. dosage for Benicar (Figure 1),
about 90% of the ultimate pressor effect can be achieved with only 40mg, once per day. But this dose is not well tolerated by sarcoid patients. Partly this may be the result of the additional Angiotensin receptors in the inflamed tissues [8], all of which have to be blocked, and partly it may be due to an increased production of serum ACE by macrophages in response to the partial blockade. Sarcoid patients experience symptoms ranging from increased fatigue to psychedelic dreams [22] when prescribed ARBs just once daily, the customary prescription for hypertension
To be fully effective, we found that Benicar must be prescribed to sarcoidosis patients as 40mg every 6 to 8 hours. We found the ARB Diovan (Valsartan) to be less effective than Benicar, but it may be used at the 80mg q8h described in our earlier paper [22]. Two patients reported sinus congestion with the Diovan blockade, which was not present after changing to Benicar. Controlled studies are needed to accurately define the blockade capability of each ARB, individually and in combination.
Implications of the Hypothesis
The blockade of A-II Type 1 receptors has been shown (in-vitro) to reduce production of the Th1 cytokines [12], including TNF-α [25], interdicting the inflammatory process. This confirms the benefits that were observed by our subjects using Angiotensin Blockade, and adds to the likelihood that blockade may prove to be an effective alternative therapy for Sarcoid inflammation, one which does not involve the use of corticosteroids.
Corticosteroids, the customary treatment for sarcoidosis, exert anti-inflammatory properties by inhibiting the activation of NF-κB [14]. But this inhibition also stops NF-κB from performing other, desirable, tasks, such as signaling the differentiation and activation of osteoclasts in the bone marrow. Even though ARBs block Angiotensin II’s stimulation of NF-κB and suppress the production of the inflammatory cytokines [12] they act through an entirely different pathway, and we expect the complications which might arise from their anti-inflammatory use to be mild, similar to those experienced by patients using ARBs to treat hypertension.
This hypothesis does not require that an immune ‘hyper-sensitivity’ exists in Sarcoidosis. The granuloma of sarcoidosis may well result from an uncontrolled proliferation of the macrophage biochemistry in response to an otherwise normal immune reaction.
It has been 53 years since Guy Scadding identified that the ability to tolerate exogenous Vitamin D was correlated with the prognosis of his sarcoid patients [17].
Yet in the succeeding years, clinical research has largely ignored the possibility that the active hormone of the Vitamin D metabolism, 1,25-D, might profoundly affect the inflammatory state. Even today, the test for serum 1,25-D is rarely performed unless hypercalcemia or hypercalciuria are manifest. Nevertheless 1,25-D is a secosteroid hormone with systemic actions which are independent of any hypercalcemic or hypercalciuric state.
We identified many normocalcemic patients where treatment involving isolation from sunlight, the use of medical eyeshields, and reduction of dietary vitamin D intake, were sufficient to stabilize or remit the symptoms of sarcoidosis.
The experiences of the one patient, who decided to more fully document her suffering, provide data that cannot be ignored. An analysis of her D metabolites, serum Calcium, serum ACE, and Alkaline Phosphatase can leave no doubt that exposure of this sarcoidosis patient to normal and customary levels of sunlight caused regression of the disease state, and isolation alleviated the disease state. There has never been a controlled study of the role of 1,25-D in the prognosis, or treatment, of sarcoidosis, and our observations clearly indicate that this needs to be rectified without delay.
The D-Ratio subjectively correlated with severity of systemic inflammation, both in the cohort as a group, and in the volunteer patient.
Our “Angiotensin Hypothesis” describes a biochemical mechanism through which extra-renally produced 1,25-D feeds the proliferation of sarcoid inflammation. It explains why sarcoid patients are sensitive to exogenous vitamin D, and especially sensitive to sunlight.
It also explains why Angiotensin Receptor Blockade is proving an effective treatment for fatigue, and other common symptoms of sarcoidosis.
Surprisingly, not all the currently available angiotensin receptor agonists are equally effective at delivering symptomatic relief. Controlled trials need to be instituted in order to define these differences and to identify optimized ARB therapies.
5. Competing Interests
None Declared. The study was fully funded by the authors.
6. Authors’ Contributions
Trevor G. Marshall is a Guarantor of this study, and contributed original research, writing, reviewing and final approval of the text. Frances E (Liz) Marshall is also a Guarantor, and contributed original research, reviewing and final approval of the text
7. Acknowledgements
Written consent was obtained from each patient for publication of any data that is not on the public record
