ELECTROPHYSIOLOGY: FIGURE 2, FIGURE 3 AND FIGURE 4
FOOTNOTES AND ACKNOWLEDGMENT
REFERENCES, LETTERS TO EDITOR AND SUPPLEMENT
MISCELLANEA AND KEY TAKE-HOME MESSAGES
AMYLOID PLAQUE ( AND NOT DIFFUSE AMYLOID ) IS A CONDITION FOR NEURONAL DYSFUNCTION
Alexei R. Koudinov,a,CA Temirbolat T. Berezova and Natalia V. Koudinovaa,b
a Berezov Academic Laboratory, Russian Academy of Medical
Sciences, Timoshenko St., 38-27, Moscow, 121359 Russia;
b Weizmann Institute of Science, Department of Biological
Regulation, Rehovot, 76100 Israel.
First submitted: December 23, 1999, Published online: December, 2001
| AUTHORS KEY ARTICLE 1 AND KEY ARTICLE 2 |
|
RESULTS
AND DISCUSSION: B.ELECTROPHYSIOLOGY
ELECTROPHYSIOLOGY: FIGURE 2, FIGURE 3 AND FIGURE 4 FOOTNOTES AND ACKNOWLEDGMENT REFERENCES, LETTERS TO EDITOR AND SUPPLEMENT MISCELLANEA AND KEY TAKE-HOME MESSAGES |
Please remember about your browser shortcuts: to navigate back and forth use <CTRL><LEFT> and <CTRL><RIGHT> keys on your keyboard.
Todate, it is not established whether the maturation of brain amyloid deposits, particularly the development of Congo Red positive neuritic plaques, is an essential event leading to neuronal dysfunction. Recent study by Naslund et al. [ 8 ] attempted to correlate the amyloid load with the cognitive decline and the severity of dementia in Alzheimer disease patients. However, the latter report estimated 55% and 40% of the study subjects without detectable plaques as the ones having readily detectable levels of "Ab(x-42) and Ab(x-40)" . In addition, recent reports have suggested the possible importance of factors other then brain amyloid in Alzheimer's neuronal abnormality (oxidative stress [ 9WEB+ ] or lipid metabolism [ 10WEB+, 11 ] disbalance, for example) and behavioral disturbances without amyloid deposits in mice overexpressing human APP with Flemish and Dutch mutations [ 12 ], keeping open the question on the role of neuritic plaques in the genesis of Alzheimer's neuronal dysfunction.
In our study, we utilized hippocampal slices of 16.5 and 25.5 month old transgenic mice expressing nonmutated human APP695 [ 13 ], and age-matched non-transgenic wild type control mice. We aimed to differentiate the separate actions of diffuse and plaque amyloid on hippocampal synaptic plasticity using immunohistochemical analysis, Congo Red staining, amyloid extraction and extracellular recording of CA1 field excitatory postsynaptic potentials ( fEPSPs ). The results indicate that amyloid plaque (and not diffuse amyloid) may represent one of the possible causes of neuronal dysfunction and synaptic plasticity failure.
Animal care and tissue collection
Transgenic and wild type mice of 16.5 and 25.5 months age groups (n=6) were maintained on the standard diet at the animal facility of the Weizmann Institute of Science, Rehovot, Israel. All experimental procedures were in accord with National Institutes of Health guide for the use of laboratory animals. Two or three hippocampal slices from each mouse were subjected to both electrophysiology and to immunohistochemistry.
Additionally, two transgenic and two wild type mice at the age of 16.5 months were subjected to transcardial perfusion, followed by thin sectioning of the brain and immunohistochemical analysis. In selected experiments, the hippocampal slices of 16.5 month old transgenic and wild type mice were subjected to the biochemical analysis of Ab.
Ex-vivo hippocampal slices
Hippocampal slices were prepared and electrophysiological analysis was performed essentially as we described previously [ 10, 14 ]. Briefly, after mice decapitation the hippocampus was rapidly removed and placed into cold (20C) artificial cerebrospinal fluid (ACSF, pH 7.4 containing (in mM): 124 NaCl, 2.0 KCl, 1.24 KH2PO4, 2.0 MgSO4, 2.5 CaCl2, 26 NaHCO3 and 10 D-glucose) saturated with 95 % O2 / 5% CO2 (flow rate 0.4 l/min) and adjusted with sucrose (7g per 600 ml of ACSF) to 320 mOsm osmolarity. The hippocampal slices (400 m) were prepared with a McIlwain tissue slicer, USA. The slices were incubated in a recreation chamber at room temperature (250C) for 1.5 h in ACSF.
Electrophysiology
The slices were transferred to a recording chamber (held at constant temperature of 320C) and submerged slices were superfused with ACSF at a flow rate of ~1.5 ml/min. Extracellular electrodes (~4 MW, 0.75 mM NaCl) were guided by micromanipulator into stratum radiatum of CA1 (200 m deep) under binocular. Bipolar (A. Koudinov fabricated) tungsten (50 m wire size) stimulating electrode was also placed into CA1 stratum radiatum. The stimulations were delivered every 30 s at 50 ms pulse duration yielding fEPSP waveforms. After stable baseline responses were established and input /output (I/O) curve values recorded, tetanus induced LTP was induced by delivering a 100 Hz, 1 sec stimuli train through the stimulation electrode at the baseline test stimulus intensity. For long term depression (LTD) study slices were stimulated every half a second (2 Hz) for 5 min at the test stimulus intensity.
Data were collected, stored and analyzed on a PC using Asyst 3.1 and GraphPad Prizm 2.0 data acquisition and analysis software. The I/O relationship, LTP and LTD were expressed as a fEPSP amplitude and slope change versus stimulus intensity and time, respectively. Data were normalized with respect to the steady baseline values and expressed as mean±SEM. Non-parametric unpaired Mann-Whitney test was used for determining significant differences between potentiation/depression levels of trangenic and wild type slices at the indicated time points. A probability of 0.05 (one tailed) or less was accepted as statistically significant.
Immunohistochemistry and Congo Red staining for amyloid
The hippocampal slices from 25.5 month old mice used for synaptic plasticity study, were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS), pH=7.4 for 72 hrs [ 10, 14 ]. We also used hippocampal sections from 16.5 month old mice killed by transcardial perfusion with PBS and 4% paraformaldehyde in PBS [ 11, 14 ]. For immunohistochemistry, 400 m-thick fixed slices were cutted with a microtome to a 40 m sections. Free floating sections were washed in PBS (2x) and then incubated with 3% H202 (prepared on PBS, containing 10% methanol) for 20 min to remove endogenous peroxidase activity. Sections were then washed (4x 10 min each) with PBS and blocked for 24 hrs at 40C in 10% fetal calf serum and 1% glycine in PBS (blocking solution), followed by 14 hrs incubation with 4G8 or 6E10 (1:1000, Senetek, PLC.) monoclonal anti-Ab antibodies in the blocking solution at 40C. Tissue sections were washed (5x 40 min each) in blocking solution to remove unreacted primary antibodies. Secondary biotinylated goat anti-mouse antibody (1:750) were added for 1.5 h at 250C, followed by washing with the blocking buffer (3x 40 min each). After washing, ABC solution (Vector Elite kit, 1:300 of reagents A and B in blocking solution) was added for 40 min, followed by section washing in blocking solution and then in PBS (2x 10 min each). For visualization, immunostained sections were reacted with 3',3-diaminobenzidine tetra-hydrochloride (Sigma Tablet kit, USA) and washed in PBS.
For Congo Red staining thin sections were stained with 0.5% Congo Red in 50% alcohol for 30 min, followed by one minute treatment with 0.2% KOH in 80% alcohol and water. Sections were mounted on gelatinized slides, air dried, dehydrated in serially diluted ethanol (50, 70, 90, 95 and 100%), cleared with Xylenes, and coverslipped with cover glass and Permount, USA.
To control the specificity of 4G8 and 6E10 immunostaining, antibody solutions were preadsorbed with the access of synthetic peptide Ab1-40 (1 mg /0.5 ml) prior to the incubation with the sections.
Fluorescence of plaque-like amyloid labeled by Congo Red was obtained with 488 nm of excitation using a confocal microscope LSM 510 (Zeiss, Germany) equipped with an argon laser [ 1S ].
Amyloid extraction
Hippocampal slices were homogenized with the cold PBS (0.5 ml per 20 mg of tissue) containing protease inhibitors [ 15 ], and subjected to centrifugation in a Beckman TiL 100.2 rotor at 100,000 g for 3 hrs at 40C. The supernatant fraction and the pellet were dialyzed against water with 1,000 Da cut-off membrane (Spectrum, USA), lyophilized and subjected to Ab extraction with 20% and then with 80% acetonitrile in 0.1% trifluoroacetic acid [ 16 ]. Both acetonitrile soluble fractions were combined, lyophilized and subjected to 13% TRIS-Tricine SDS/PAGE [ 17, 18 ] and immunoblot analysis on Immobilon P membranes (Wattman, USA) with 6E10 and 4G8 anti-Ab monoclonal antibodies and with the monoclonal antibody against APP (Zymed, USA), followed by ECL (Amersham) essentially as previously reported [ 15, 18 ].
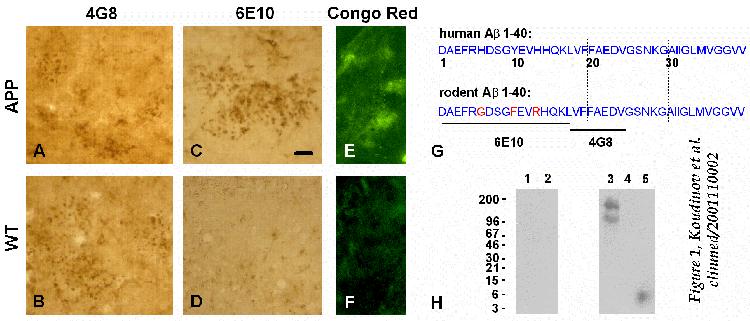
A. IMMUNOHISTOCHEMICAL ANALYSIS: AMYLOID AS ALZHEIMER’S HALLMARK
Immunohistochemistry of slices from aged animals (25.5 months) with 4G8 and 6E10 antibodies (anti-human/mouse-Ab and anti-human-Ab, respectively, see antibody specificity scheme, Figure 1G ) revealed extracellular (staining with no triton) hippocampal immunoreactivity of mouse Ab in transgenic mice (Fig. 1A) and in wild types (Fig. 1B), and verified extracellular deposits of human Ab in the transgenic mice hippocampus (Fig. 1C). Essentially identical results were obtained under the condition of membrane permeabilization with 0.1% Triton X-100 in the blocking buffer (not shown). The specificity of immunostaining was confirmed by the preadsorption of antibodies with the access of synthetic peptide Ab(1-40).
Staining for amyloid showed Congo Red fluorescence specifically in aged transgenic mice (and not in aged wild type mice) hippocampal sections (Fig. 1E). Congo red stains specifically amyloid plaques (but not diffuse amyloid) due to the binding to the b-pleated sheet secondary structure of Ab protein in amyloid fibrils [ 1, 2 ]. The latter observation indicates that expression of non-mutated human Ab in aged transgenic mice leads to a mature Alzheimer's plaque-like amyloid and that Ab deposits in wild type mice have a nonmature diffuse nature.
In contrast to the 25.5 month aged animals, the 16.5 month old transgenic and wild type mice expressed neither human nor mouse Ab immunoreactivity (not shown). To confirm this observation we analyzed 16.5 month old transgenic and wild type mice for soluble and aggregated Ab by immunoblot analysis of the acetonitrile extracts of ultracentrifugation supernatant- and pellet-fractions of hippocampal homogenates. We did not recover human transgenic (Fig. 1H) or mouse (not shown) Ab immunoreactivity in the transgenic and wild type hippocampus. However, anti-human Ab antibody 6E10 labeled a protein of high molecular weight (in the range of 96 to 200 kDa) in the trangenic mice. This protein band was also stained with the antibody against APP aminoterminus (not shown) confirming human APP expression in the transgenic hippocampus.
 You may need to resize your browser
window for better figure view.
You may need to resize your browser
window for better figure view.
Immunochemical analysis of APP transgenic mice.
A-D, Comparison of extracellular Ab
immunoreactivity in aged (25.5 months) human non-mutated APP695
transgenic mice and age-matched non-transgenic wild type control mice hippocampus.
Immunohistochemistry was performed with no triton and 4G8 and 6E10 monoclonal
antibodies (1:1000). The presented fields are CA1 areas of ex-vivo
slices from the batches used for extracellular recording; Bar, 20
m.
Similar Ab pattern was observed in the dentate
gyrus (not shown). Alzheimer's-like plaque amyloid in transgenic mice (E),
versus wild type mice (F), was visualized by confocal fluorescent
microscopy of Congo Red stained sections; Bar, 100 m.
(H) 16.5 month old wild type (control, lanes 1 and 2) and transgenic
mice (lanes 3 and 4) were analyzed for the soluble and/or aggregated human
Ab in the acetonitrile extracts of the supernatant
(lanes 1 and 3) and pellet (lanes 2 and 4) fractions of the hippocampal
homogenates, respectively, by immunoblot analysis with 6E10 and 4G8 (not
shown) monoclonal antibody. While there were no detectable levels of Ab
present in transgenic hippocampus, we recovered 6E10-positive high molecular
weight human APP immunoreactivity in the supernatant fraction (lane 3),
and confirmed it by immunostaining with the antibody against APP aminoterminus.
Lane 5, 5 ng of synthetic Ab1-40 (positive control).
Molecular weight markers (in kDa) are shown on the left. Scheme (G)
represents human and rodent Ab1-40 amino acid
sequence differences and the sequence specificity of anti-Ab
monoclonal antibodies used in this study.
B. ELECTROPHYSIOLOGICAL
ANALYSIS: PLAQUE AMYLOID AND SYNAPTIC PLASTICITY![]()
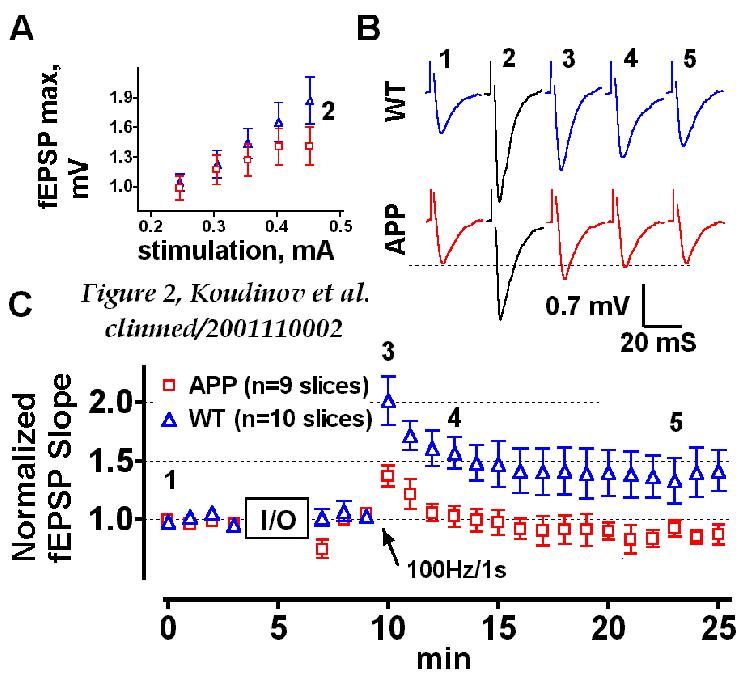
Electrophysiological analysis revealed that 25.5 month old transgenic mice had lower input-stimulus/output-response (I/O) characteristics (Figure 2A). It is generally accepted that in the CA1 area of the hippocampus EPSP consists of two major elements that depend on the activation of N-methyl-D-aspartate (NMDA) and non-NMDA (mainly AMPA) subtypes of ionotropic glutamate receptors [ 19WEB+ ]. The 50 ms stimulation pulse duration (employed here to evoke fEPSP) generates significant AMPA responses and it is thus possible that this particular component of the EPSP is responsible for lower I/O characteristics of aged transgenic mice [ 19WEB+ ].
Aged transgenic mice were impaired in both the amount of initial post-tetanic potentiation (137.2±9.3%, n=9, against 201.8±20.8% of the wild type, n=10, p=0.014) and in the maintenance of the LTP, sampled 4 smin (99.53±9.78%, n=9, and 148.6±15.01%, n=10, p=0.0318) and 10 min (92.64±7.02%, n=9, and 133.2±9.29%, n=10, p=0.0357) after the tetanic stimulation. There were no significant differences in induction (p=0.2403) and maintenance (p=0.0649) of the LTP in wild type slices taken from 16.5 and 25.5 months old mice (Fig. 2 and Figure 3A) despite the development of diffuse mouse Ab deposits in wild type hippocampus over the indicated age (indicating the lack of importance of diffuse amyloid for synaptic plasticity failure). On the other hand, transgenic slices expressing plaque-like amyloid at age 25.5 months showed a significant decline in both induction (p=0.0047) and maintenance (p=0.0048) of the LTP compared to the 16.5 month old mice.
 You may need to resize your browser
window for better figure view.
You may need to resize your browser
window for better figure view.
Electrophysiological analysis of aged (25.5 months) APP transgenic
mice
(A) Input/output (I/O) relationship in APP transgenic
(squares) and wild type non-transgenic (WT, triangles) hippocampal slices.
(B) Field synaptic responses obtained at the baseline (1) and high
stimulus intensity (2) recordings as well as immediately after (3) and
3 (4) and 13 min after (5) the high-frequency train of stimuli. (C)
Impairment of tetanic LTP in CA1 of ex-vivo slices from APP transgenic
versus WT hippocampus. In all cases n=9 and n=10 slices for APP transgenic
and WT mice, respectively. Arrow indicates time of tetanus.
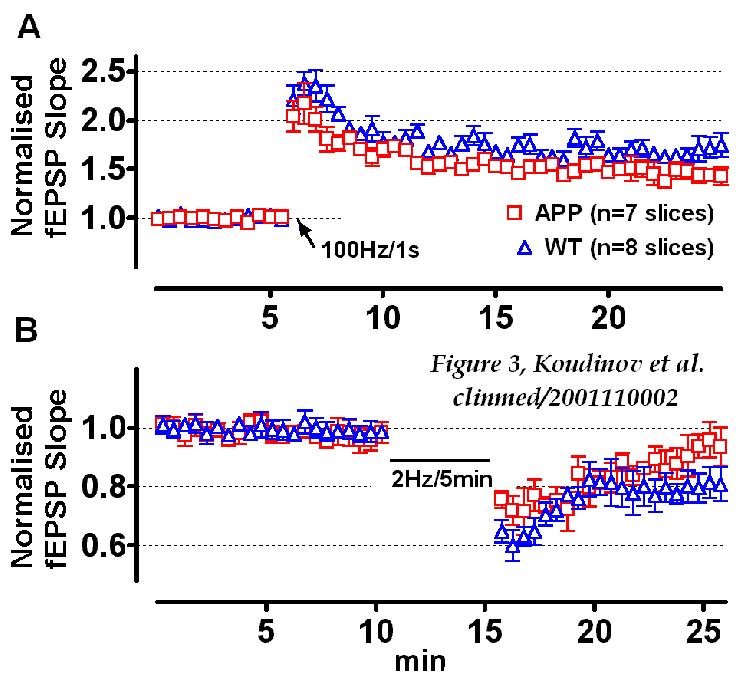
In contrast to the aged 25.5 month old mice, transgenic mice of the younger age (16.5 months) expressed no amyloid and did not differ from the wild type mice in the induction and maintenance of the LTP (Fig. 3A). There were, however, differences in the LTD (Fig. 3B), another important parameter of neuronal plasticity. It was shown previously [ 20 ] that bath application of the soluble APP (100 nM, 1 h) adsorbed the ability of rodent hippocampal slices to maintain LTD. It is thus conceivable that mild modulation of the hippocampal LTD in adult transgenic mice (16.5 months) is due to the human APP expression in the transgenic mice hippocampus (Fig. 1H). Another study by Larson et al. [ 21 ], however, suggests a more striking modulation of hippocampal physiology by human mutant (V717F)APP in transgenic mice at age 4-5 months. Although the experimental protocol of this report (specifically, maintaining slices at 360C; differences in the media recipe, particularly including ascorbate, known to modulate EPSP [ 22 ] in ACSF) does not match the one employed here and in the above cited report by Ishida et al. [ 20 ], it indicates that APP mutations (yet representing very small cohort of all Alzheimer disease cases) may exacerbate additional abnormalities in synaptic and behavioral plasticity "prior to the formation of amyloid beta peptide deposits." The transgenic mice that we used in this study mild overexpress non-mutated human APP and in our view offer more relevant system to model non-mutated human APP expression [ 13 ].
 You may need to resize your browser
window for better figure view.
You may need to resize your browser
window for better figure view.
Electrophysiological analysis of 16.5 month old APP transgenic mice
APP transgenic and wild type non-transgenic (WT) mice expressed similar tetanus (arrow) induced LTP (A) and were different in the amount of the long term depression (B). However, depression values probed 10 min after the low frequency stimulation sequence did not reach statistical significance (98.95±10.34%, n=7, and 79.3±12.8%, n=6 in the APP-TG and WT, respectively, p=0.0625, one-tailed).
Two major components contributing to the tetanus induced LTP are NMDA LTP and non-NMDA (dependent on a rise in intracellular calcium concentration) LTP [ 23WEB+ ]. Fast onset NMDA- and developing slow non- NMDA-LTP can be isolated by using a specific tetanic stimulation paradigm in the presence of 30 mM nifedepine, a blocker of voltage-gated calcium channels, and 25 mM D,L-2-amino-5-phosphonovaleric acid, an NMDA antagonist, respectively [ 23WEB+, 24 ]. Moreover, another type of slow onset LTP was described, a muscarinic LTP, which can be evoked by the application of 0.25-2.0 mM carbachol in the absence of tetanic stimulation [ 23WEB+, 24 ]. Non-NMDA-LTP and muscarinic LTP share similar lack of expression in adult transgenic mice expressing human Cu/Zn-superoxide dismutase, SOD1 [ 24 ] in very old (24-30 months) and in adult Wistar rat hippocampal slices treated with low (~30 mM) dose of H2O2 [ 23 ].
Regardless of the deficit of specific receptor machinery hippocampal slices from aged transgenic mice may be different from wild type controls in their ability to regulate second messenger pathways and/or generate the action potential. Thus, transgenic mice may be impaired in the phosphorylation of the nuclear cAMP responsive element binding protein (abbreviated as CREB), modulated by micromolar concentrations of Ab [ 25 ] and known as a necessary event in neuronal plasticity [ 26 ]. Transgenic mice may be also impaired in metabotropic glutamate receptors (mGluR) and associated second messenger machinery, activation which was shown to be coupled to the APP processing [ 27 ] and is essential for the priming of the LTP [ 28 ]. It is also possible that in transgenic mice neurons are depolarized relative to the control slices, yielding reduction of their fEPSPs and impairment of their ability to express larger fEPSPs following tetanic stimulation.
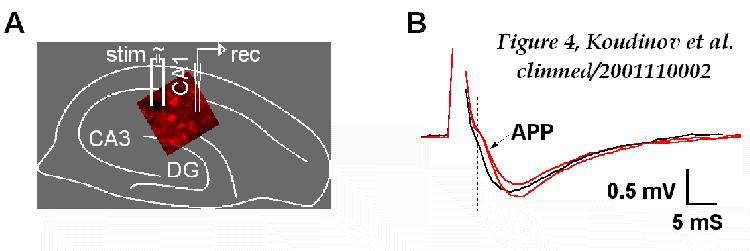
Finally, transgenic mice may have deteriorated neuronal spike propagation machinery due to the tunneling amyloid plaques (Figure 4A). This is supported by the study on the disruption of neural networks in Alzheimer's disease [ 29 ]. This report modeled the electrophysiological effect of the changed neuronal processes that cross through Ab plaque deposit and foretold the delay of several milliseconds over an average plaque. Our comparison of individual fEPSPs revealed this predicted change of few milliseconds in initial post-tetanic traces in transgenic versus wild type slices (Fig. 4B), confirming importance of plaque amyloid for neuronal spike propagation.
 You may need to resize your browser
window for better figure view.
You may need to resize your browser
window for better figure view.
Schematic matching of the positioning of the recording (rec) and stimulating (stim) electrodes
for the employed in the study extracellular recording in the CA1 and the Congo Red fluorescence, observed in the APP transgenic hippocampal slice (A). (B) Individual fEPSPs recorded after the high frequency (100 Hz/1 s) train of stimuli revealed 1.5-2.0 msec delay in the onset of the evoked synaptic responses in the APP transgenic (arrow) compared to the wild type non-transgenic slices.
Our data provide evidence that one of the possible causes of Alzheimer's-like neuronal dysfunction and synaptic plasticity deficit is senile plaque (and not diffuse) amyloid. Our data are in dispute with the paper in JAMA [ 8 ] and the accompanying commentary [ 30WEB+ , see also 31WEB+ ] proposing that Ab peptide not incorporated into histologically visible plaques is an early neurochemical hallmark of dementia [ 2S, 3S ].
Our results also suggest that in Down syndrome (characterized by diffuse amyloid deposition in early life) and in pre-plaque stages of Alzhemer disease, the other factors (such as brain cholesterol and other lipid metabolism misregulation [ 10WEB+, 11 , LE1, LE2, LE3, LE4, LE5 ] or oxidative stress disbalance [ 9WEB+, 24 ]) may contribute to the neuronal and behavioural [ 2S, 3S ] dysfunction.
The precise molecular mechanism of amyloid-plaque-mediated synaptic plasticity deficit, however, remains to be investigated.
While this paper was under submission another key contribution on this subject was published in December 2000 [ 4S ]. We are indebted to Sh. Halav and J. Hermesh for excellent animal care. See Ref. 10 for support and funding itemization. The preliminary account of this report has been published in the abstract form and presented at the 10th Meeting of the European Neurological Society, 18-21 June 2000, Jerusalem, Israel [ 5S ], and at World Alzheimer congress 2000, Washington, DC, July 9-12, 2000 [ 6S ]. The earlier version of this article was first submitted as research letter (for letter text see [ 5S ]) during December 23, 1999. In the present form this contribution was submitted to Science, J Neurosci and to Neurosciences. Our research is dedicated to our parents.
FOR
YOUR CONVENIENCE:
|
THIS
ARTICLE IS BASED ON:
|
|
|
1. Koudinova NV, Berezov TT, Koudinov AR. Amyloid beta: ALzheimer's disease and brain beta amyloidoses. Biochemistry (Moscow) 64, 752-757 (1999) [ PubMed ] [ Reprint Order ].
2. Koudinov AR, Koudinova NV, Berezov TT, Ivanov YD. HDL Phospholipid: a natural inhibitor of Alzheimer's amyloid b fibrillogenesis? Clin Chem Lab Med. 37, 993-994 (1999) [ PubMed ] [ Abstract ] [ Reprint Order ].
3. Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173-177 (1999) [ PubMed ].
4. Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castano EM, Frangione B. Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nature Med. 4, 822-826 (1998) [ PubMed ].
5. Chapman PF, White GL, Jones MW, et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nature Neurosci. 2, 271-276 (1999) [ PubMed ].
6. ( 3 web+ citations ) Rioult-Pedotti M-S, Friedman D, Donoghue JP. Learning-induced LTP in Neocortex. Science 290, 533-536 (2000) [ PubMed ]; Bliss TVP, Collingridge JL. A synaptic model of memory: long term potentiation in the hippocampus. Nature 361, 31-39 (1993) [ PubMed ]; Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science 285, 1870-1874 (1999) [ PubMed ].
7. Nalbantoglu J, Tirado-Santiago G, Lahsaini A, et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature 387, 500-505 (1997) [ PubMed ].
8. Naslund J, Haroutunian V, Mohs R, et al. Correlation between elevated levels of amyloid b-peptide in the brain and cognitive decline. JAMA. 283, 1571-77 (2000) [ PubMed ].
9. ( 5 web+ citations ) Perry G, Nunomura A, Hirai K, Takeda A, Aliev G, Smith MA. Oxidative damage in Alzheimer's disease: the metabolic dimension. Int J Dev Neurosci. 18, 417-421 (2000) [ PubMed ]; Kontush A. Amyloid-beta: an antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer's disease. Free Radic Biol Med. 31, 1120-1131 (2001) [ PubMed ]; Nunomura A, Perry G, Pappolla MA, et al. Neuronal oxidative stress precedes amyloid-beta deposition in Down syndrome. J Neuropathol Exp Neurol. 59, 1011-1017 (2000) [ PubMed ]; Nunomura A, Perry G, Aliev G, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 60, 759-767 (2001) [ PubMed ]; Gahtan E, Auerbach JM, Groner Y, Segal M. Reversible impairment of long-term potentiation in transgenic Cu/Zn-SOD mice. Eur J Neurosci. 10, 538-544 (1998) [ PubMed ].
10. ( 5 web+ citations ) Koudinov AR, Koudinova NV. Essential role for cholesterol in synaptic plasticity and neuronal degeneration. FASEB J. 15, 1858-1860 (2001), published online June 27, 2001, 10.1096/fj.00-0815fje [ PubMed Citation ] [ Full Text ] [ Authors Preface ]; Koudinova NV, Koudinov AR, Yavin E. Alzheimer’s Ab1-40 peptide modulates lipid synthesis in neuronal cultures and intact rat fetal brain under normoxic and oxidative stress conditions. Neurochem Res. 25, 653-660 (2000) [ PubMed ] [ Abstract ] [ Reprint order ]; Eckert GP, Wood WG, Muller WE. Effects of aging and beta-amyloid on the properties of brain synaptic and mitochondrial membranes. J Neural Transm. 108, 1051-1064 (2001) [ PubMed ]; Michikawa M, Gong JS, Fan QW, Sawamura N, Yanagisawa K. A novel action of Alzheimer's amyloid beta-protein (Abeta): oligomeric Abeta promotes lipid release. J Neurosci. 21, 7226-7235 (2001) [ PubMed ]; Chochina SV, Avdulov NA, Igbavboa U, Cleary JP, O'Hare EO, Wood WG. Amyloid beta-peptide(1-40) increases neuronal membrane fluidity. Role of cholesterol and brain region. J Lipid Res. 42, 1292-1297 (2001) [ PubMed ].
11. Koudinov AR, Koudinova NV. Brain Cholesterol Pathology is the Cause of Alzheimer's Disease. Clin Med Health Res. published online November 27, 2001, clinmed/2001100005, http://clinmed. netprints.org/cgi/content/full/2001100005v1 [ Full Text ] [ Authors Preface ] [ Letter to the Editor ].
Cited above recent key article discusses the role for cholesterol, amyloid b and tau in synaptic plasticity, neurodegeneration and Alzheimer's disease.
12. Kumar-Singh S, Dewachter I, Moechars D, et al. Behavioral disturbances without amyloid deposits in mice overexpressing human amyloid precursor protein with Flemish (A692G) or Dutch (E693Q) mutations. Neurobiol Dis. 7, 9-22 (2000) [ PubMed ].
13. Lamb BT, Sisodia SS, Lawler AM, et al. Introduction and expression of the 400 kilobase precursor amyloid protein gene in transgenic mice. Nature Genet. 5, 22-29 (1993) [ PubMed ].
14. Friedman LK, Koudinov AR. Unilateral GluR2(B) Hippocampal Knockdown: A Novel Partial Seizure Model in Young Rat. J Neurosci. 19, 9412-9425 (1999) [ PubMed ] [ Full Text ] [ Reprint Order ].
15. Koudinov AR, Koudinova NV. Soluble amyloid beta protein is secreted by HepG2 cells as an apolipoprotein. Cell Biol Inter. 25, 265-271 (1997) [ PubMed ] [ Reprint Order ].
16. Kaplan B, Haroutunian V, Koudinov AR, Patael Y, Pras M, Gallo G. Biochemical assay for amyloid b deposits to distinguish Alzheimer's disease from other dementias. Clin Chim Acta 80, 147-159 (1999) [ PubMed ] [ Reprint Order ].
17. Koudinov AR, Berezov TT, Koudinova NV. The levels of soluble amyloid beta in different high density lipoprotein subfractions distinguish Alzheimer's and normal aging cerebrospinal fluid: implication for brain cholesterol pathology? Neurosci Lett. 314, 115-118 (2001) [ PubMed ] [ Full Text ] [ PDF ] [ Reprint Order ].
The above article presents experimental data and discusses how an impairment of extracellular lipoprotein-mediated trafficking of brain cholesterol may be linked to synaptic function, neural apolipoproteins and Alzheimer’s disease
18. Koudinov AR, Koudinova NV, Kumar A, Beavis R, Ghiso J. Biochemical characterization of Alzheimer's soluble amyloid beta protein in human cerebrospinal fluid: association with high density lipoproteins. Biochem Biophys Res Commun. 223, 592-597 (1996) [ PubMed ] [ Reprint Order ].
19. ( 1 web+ citations ) Astrelin AV, Sokolov MV, Behnisch T, Reymann KG, Voronin LL. Principal component analysis of minimal excitatory postsynaptic potentials. J Neurosci Meth. 79, 169-186 (1998) [ PubMed ]; Samestkij EA, Bayazitov IT, Kleschnikov AM. AMPA and NMDA receptor mediated components of "minimal" EPSPs recorded from the same synaptic terminals show equal posttetanic LTP in the CA1 hippocampal region in vitro. 5th IBRO World Congress of Neuroscience, 11-15 July, 1999, Jerusalem, Israel. Abstract book p. 191 (1999).
20. Ishida A, Furukawa K, Keller J, Mattson MP. Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD, and enhances LTP in hippocampal slices. Neuroreport 8, 2133-2137 (1997) [ PubMed ].
21. Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 840, 23-35 (1999) [ PubMed ].
22. Xie Z, Sastry BR. Induction of hippocampal long-term potentiation by alpha-tocopherol. Brain Res. 604, 173-179 (1993) [ PubMed ].
23. ( 1 web+ citations ) Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 17, 8695-8701 (1997) [ PubMed ]; Cavus I, Teyler T. Two forms of long-term potentiation in area CA1 activate different signal transduction cascades. J Neurophysiol. 76, 3038-3047 (1996) [ PubMed ].
24. Koudinov A, Groner Y, Segal M. Cu/Zn-SOD transgenic mice are impaired in slow onset, long term potentiation. Neurosci Lett. 51, S23 (1998) [ Abstract ] [ Presentation Order ].
25. Sato N, Kamino K, Tateishi K, et. al. Elevated amyloid b protein (1-40) level induces CREB phosphorylation at serine-133 via p44/42 MAP kinase (Erk1/2)-dependent pathway in rat pheochromacytome PC12 cells. Biochem Biophys Res Commun. 232, 637-642 (1997) [ PubMed ].
26. Segal M, Murphy DD. CREB activation mediates plasticity in cultured hippocampal neurons. Neural Plast. 6, 1-7 (1998) [ PubMed ].
27. Nitsch RM, Deng A, Wurtman RJ, Growdon JH. Metabotropic glutamate receptor subtype mGluR1alpha stimulates the secretion of the amyloid beta-protein precursor ectodomain. J Neurochem. 69, 704-712 (1997) [ PubMed ].
28. Cohen AS, Raymond CR, Abraham WC. Priming of long-term potentiation induced by activation of metabotropic glutamate receptors coupled to phospholipase. Hippocampus 8, 160-170 (1998) [ PubMed ].
29. Knowles RB, Wyart C, Buldyrev SV, et al. Plaque-induced neurite abnormalities: implications for disruption of neural networks in Alzheimer's disease. Proc Natl Aca Sci USA 96, 5274-5279 (1999) [ PubMed ].
30. ( 4 web+ citations ) Selkoe D. The origins of Alzheimer disease: a is for amyloid. JAMA. 283, 1615-1617 (2000) [ PubMed ] [ Full Text ]; Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 81, 741-66 (2001) [ PubMed ]; Selkoe DJ. Toward a comprehensive theory for Alzheimer's disease. Hypothesis: Alzheimer's disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Ann N Y Acad Sci. 924, 17-25 (2000) [ PubMed ]; Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease Nature. Neurological disorders. 399 (Supplement), A23-A31.
To moderate the opinion expressed in cited above redundant articles ( Ref. 30 ) please read contributions referenced below
31. ( 3 web+ citations ) Joseph J, Shukitt-Hale B, Denisova NA, Martin A, Perry G, Smith MA. Copernicus revisited: amyloid beta in Alzheimer's disease. Neurobiol Aging. 22, 131-146 (2001) [ PubMed ] [ Letter to the Editor ]; Mesulam MM. (1999) Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 24, 521-529 (1999) [ PubMed ] [ Full Text ]; Lue LF, Kuo YM, Roher AE, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 155, 853-862 (1999) [ PubMed ].
RELATED
LETTERS TO EDITOR ![]()
[ Authors
"eLetters to Editor" collection ]
LE1. Koudinov AR, Koudinova NV. Alzheimer's pathogenesis: tau and amyloid - a consensus or a challenge for a third party quest ? Br Med J. E.letter published online September 4, 2001 [ Read the letter ].
LE2. Koudinov AR, Koudinova NV. Cholesterol, synaptic function and Alzheimer's disease. Br Med J. E.letter published online October 16, 2001 [ Read the letter ].
LE3. Koudinov AR, Koudinova NV. Dementia, cholesterol and the soft science of dietary fat. Br Med J. E.letter published online July 27, 2001 [ Read the letter ].
LE4. Koudinov AR, Koudinova NV. Looking forward for a historic issue, or: Is there anything besides amyloid? Br Med J. E.letter published online October 19, 2001 [ Read the letter ].
LE5. Koudinov AR, Koudinova NV. Cholesterol supply and synaptic plasticity. Submitted to Science on November 9, 2001 [ Read the letter ].
LE6. Koudinov AR, Koudinova NV. Cholesterol misregulation causes Alzheimer's disease: diet likely involved. Br Med J. E.letter published online December 1, 2001 [ Read the letter ].
1S. Jang HF. Measurement of Fibril Angle in Wood Fibres with Polarization Confocal Microscopy. J Pulp Paper Science 24, 224-30 (1998).
2S. Ferris SH, Kluger A. Assessing cognition in Alzheimer disease research. Alzheimer Dis Assoc Disord. 11S, 45-49 (1997) [ PubMed ].
3S. Franssen EH, Reisberg B. Neurologic markers of the progression of Alzheimer's disease. Int Psychogeriatr. 9 (Suppl 1), 297-306; discussion 317-21 (1997) [ PubMed ].
4S. Chen G, Chen KS, Knox J, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature 408, 975-979 (2000) [ PubMed ].
5S. Koudinov AR, Berezov TT. Alzheimer's amyloid plaque formation is a condition for neuronal dysfunction. 10th European Neurological Society Meeting, 18 June, 2000, Jerusalem, Israel. Abstract book. P534 (2000) [ Abstract ].
6S. Koudinov AR, Koudinova,
NV, Berezov TT. Is Alzheimer's amyloid plaque formation a condition for
neuronal dysfunction? Neurobiol. Aging 21, S155 (2000) [ Abstract
].
|
Readers statistics:
|
Citation example for this article:
Koudinov AR, Berezov TT, Koudinova NV. Amyloid plaque (and not diffuse
amyloid) is a condition for neuronal dysfunction. Clin. Med. Health
Res. published online December, 2001, clinmed/2001110002, http://clinmed.netprints.org/cgi/content/full/2001110002v1
You
may also wish to cite year 2000 preliminary accounts of this article
[ 5S or 6S ].