Dr I. N. Back, MA MRCGP DA
Consultant in Palliative Medicine
Holme Tower Marie Curie Centre, Penarth and Y Bwthyn Palliative Care Unit,
Pontypridd.
Correspondence to: Dr I. N. Back
Holme Tower Marie Curie Centre, Bridgeman Road, Penarth, South Glamorgan CF64
3YR
Tel: 029 2042 6000; Fax: 029 2042 6036; E-mail: ian.back@dial.pipex.com
A questionnaire was sent to senior palliative care physicians in the UK asking them to rank 12 treatments for neuropathic pain in the order they would use them. The questionnaire specified a clinical scenario of pain, and limited the treatments to twelve predefined options. 162 valid results were analysed (66% response rate). The median rank order when treating moderate pain was morphine, amitriptyline, dexamethasone, sodium valproate, TENS, diclofenac, clonazepam, flecainide, epidural or intrathecal analgesia, ketamine, methadone and ketorolac. For severe pain, epidural, ketamine, methadone and dexamethasone were ranked significantly higher, and TENS, diclofenac, sodium valproate and amitriptyline were ranked significantly lower. If lancinating pain was present, 57% would rank an anticonvulsant higher.
Neuropathic pain is common in advanced cancer, and often poses a difficult management problem for palliative care. There are many therapeutic options open to the physician faced with managing this pain, but little evidence on which to base treatment decisions. The use of tricyclic antidepressants and anticonvulsants is well established and there is much evidence from chronic benign pain supporting their efficacy.[1,2] Many other treatments have been reported, including flecainide,[3] ketamine,[4] NSAIDs,[5] alternative strong opioids (especially methadone,[6,7]) epidural or intrathecal analgesic techniques,[8] and transcutaneous electrical nerve stimulation (TENS).[9] However, most of these other treatment options have little other than case reports or anecdotal evidence supporting their use in malignant neuropathic pain. Even the place of strong opioids like morphine or diamorphine in the treatment of neuropathic pain can be questioned. [10,11] In addition, the efficacy of different treatments has not been compared in any controlled trials.
A questionnaire was designed to seek the opinion of senior doctors working in palliative care to determine the value they place on different available treatments. Three pilot questionnaires completed by consultants in palliative medicine in Wales demonstrated that such a questionnaire needed to define quite specifically the clinical situation and limit the treatment options available in order to return useful data.
The questionnaire was sent to 251 senior palliative medicine physicians, identified from a list of all consultants and medical directors who were members of the Association for Palliative Medicine for Great Britain and Ireland in October 1997. The questionnaire asked respondents to rank 12 available treatments (Box 1) in the order that they would use them to treat a patient with malignant neuropathic pain. A clinical scenario was given (shown in Box 2).
| Box 1. Treatment options. | Box 2. Clinical Scenario | |
| Amitriptyline Clonazepam Epidural or intrathecal analgesia* Dexamethasone Diclofenac Flecainide Ketamine Ketorolac Methadone Morphine/Diamorphine Sodium valproate T.E.N.S. (* assuming anaesthetist available) |
The patient is a 50-year old man with metastatic carcinoma
of the rectum. He has extensive pelvic recurrence and has received
radiotherapy a few months ago. The patient's prognosis is thought to be
between a few weeks and a few months. He is currently an in-patient and
his main problem is pain. He is complaining of pain in the anterior aspect of his left thigh (dermatome of L3/L4). The pain is continuous - burning at times, aching at times. He also describes 'pins and needles' in the same area. Sensation is present but altered (paraesthesia). No other neurological signs are elicited. The skin appears and feels normal. A recent bone scan was negative. A 'diagnosis' is made of neuropathic pain caused by infiltration or compression of the lumbo-sacral plexus or femoral nerve by tumour. Start by assuming that he has been taking regular co-codamol forte (Solpadol / Tylex) which has had no effect, and is on no other medication. |
Respondents were asked to rank all twelve treatments in the order they would use them, unless they would never use a particular treatment. They were asked to rank more than one treatment equally if 1) they would normally institute two treatments at the same time, e.g. dexamethasone and TENS, or 2) they considered two treatments to be equally effective and would choose based only on individual patient characteristics. Respondents were also asked to name one other treatment they would choose to be available in addition to the twelve offered.
The ranking was performed twice, once assuming the pain to be moderate in intensity, and then for severe pain. Moderate pain was defined as: allowing a reasonable night's sleep, not preventing normal living activities, but the major cause of the patient's distress. Severe pain was defined as: an overwhelming problem preventing any normal activities or pleasure, and the patient feels he 'cannot cope with another day like this'.
A further question asked if the presence of predominantly lancinating pain would influence their ranking of the available treatments. Lastly they were asked if they would change to an alternative opioid if the pain had responded partially to morphine / diamorphine, but the last increase in dose (e.g. from MST 30mg b.d. to 40 mg b.d.) caused hallucinations and nausea without complete pain relief.
One hundred and sixty five questionnaires were returned (66% response rate); of these, three were not completed because doctors had either retired or were not currently working in a clinical setting. Results were analysed from the remaining 162 forms.
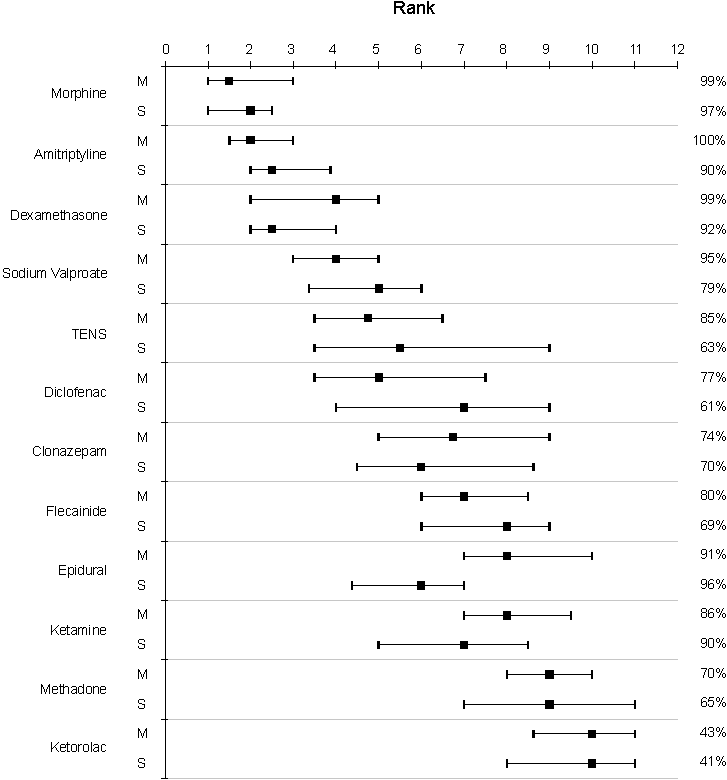
Rank order was adjusted when necessary e.g. three treatments ranked first equal were all ranked as 2, with the next treatment ranking 4. The median rank for each treatment was calculated, together with the 25th and 75th quartiles (inter-quartile range). The results are shown in Figure 1.
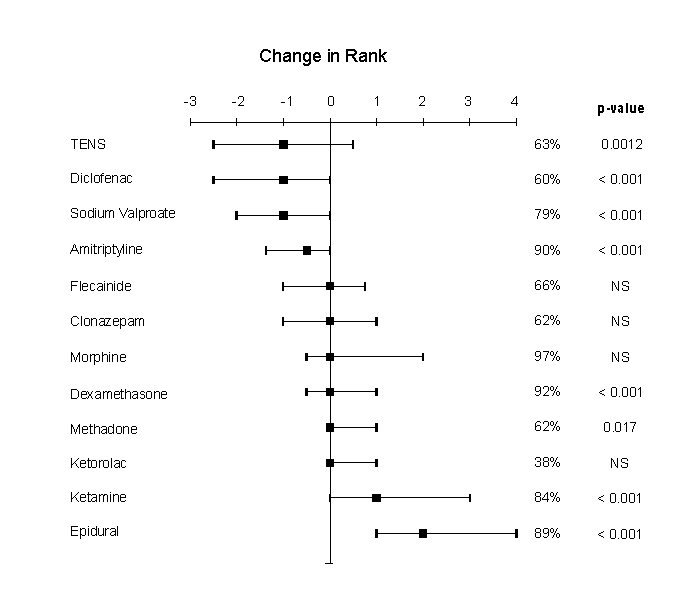
The difference between ranking of each treatment for moderate versus severe pain was calculated and the median change with inter-quartile range is shown in Figure 2. The Wilcoxon non-parametric test for paired comparison was used to compare the rank order given in moderate and severe pain. The p-values were corrected using the Bonferroni correction to allow for the fact that 12 comparisons were made. The comparison does not include those who would not use the drug in one of the situations.
Four treatments were ranked significantly lower for severe pain than for moderate pain: TENS, diclofenac, sodium valproate and amitriptyline. Four treatments were ranked significantly higher for severe pain than for moderate pain: dexamethasone, methadone, ketamine and epidural analgesia.
Side effects caused by morphine (as detailed in the question), would make 97 (60%) change to another opioid; 48 (30%) would continue with their next treatment as before. Of those who would change, several opioids were mentioned: fentanyl (40%), hydromorphone (40%), methadone (33%) and phenazocine (9%). Twenty six percent mentioned more than one alternative.
The predominant presence of 'lancinating' pain would alter the order of treatment in 101 (62%) cases (no change 46, no answer 15). Of these, 92 would prescribe an anticonvulsant earlier and 9 an antiarrhythmic earlier. Ten respondents commented spontaneously that they were aware of evidence against a preferential effect of anticonvulsants against lancinating pain; nevertheless, four of the ten would not be influenced by the evidence and prescribe an anticonvulsant earlier for lancinating pain.
When offered the chance to choose one extra treatment, 86 respondents (53%) entered an additional treatment. These additional treatments are shown in Table 1. Treatments were grouped for analysis to aid clarity. Other complementary methods mentioned were aromatherapy, massage and reflexology. Other treatments mentioned were gabapentin, clonidine, physiotherapy, psychology and bowel care.
Comments were invited at the end of the questionnaire. Many commented that they found the exercise difficult because their practice would depend considerably on individual circumstances.
First drafts of the questionnaire attempted to rank treatments for three clinical situations - namely - constant dysaesthetic pain, lancinating pain, and pain on movement. The eleven consultants helping with the pilots found the exercise too confusing, as so many hypothetical parameters were being changed.
A second questionnaire did not limit the choice of treatments but allowed any treatments to be written down in order. Two problems emerged from this: 1) doctors did not stretch themselves to consider resistant pain, instead writing down only two or three treatment options, and 2) there was difficulty in collating and comparing results in view of the large number of actual drugs that were chosen. On further discussion it was apparent that sometimes a drug within a drug 'group' was preferred because of side effects or preparations available (e.g. NSAIDs, amitriptyline and clomipramine), whilst the preference in other cases was based on a perceived difference specifically for neuropathic pain (e.g. morphine and methadone). In many cases this distinction was not clear (e.g. carbamazepine, sodium valproate and clonazepam).
The final questionnaire thus limited both the treatment options available, and
the clinical scenario to one patient in either moderate or severe pain. These
constraints limit the conclusions that can be drawn from the replies.
The median rank order of treatments shows a surprising degree of consensus, with
morphine, amitriptyline, dexamethasone and sodium valproate making the top four.
Sodium valproate was chosen as the representative of the first line anticonvulsant, as it is now probably the most commonly used in Wales. There is more evidence in the literature supporting carbamazepine than valproate, and given the chance to choose an extra treatment, 20% of respondents asked for carbamazepine even though valproate was already available to them. It is possible that this may have skewed the results, and that carbamazepine would have shown a higher rank order than valproate.
The questionnaire did not attempt to ask why choices were made. There are a number of possible reasons for what rank is given to a particular treatment including: 1) perceived efficacy of the treatment, 2) side effects or complications of the treatment, 3) speed of onset of treatment, 4) the weight of published evidence supporting the treatment. The difference in rank order seen for some treatments between moderate and severe pain was significant for a number of treatments, and may reflect a balance between these factors e.g. that the severity of pain means that a greater efficacy or speedier onset of analgesia outweigh the side effects or complications that had previously kept the treatment from being first choice. This would certainly seem to be the case for epidural analgesia.
The question about lancinating pain was asked because it is often stated that paradoxical or lancinating pain may respond better to an anticonvulsant than to a tricyclic antidepressant, but a systematic review of the treatment of neuropathic pain with anticonvulsants had concluded that there was no evidence to support this.[2] Ninety-two (57%) of the respondents nevertheless said they would use an anticonvulsant earlier, many stating specifically that this would be in preference to a tricyclic antidepressant.
In the general comments, one respondent wrote "I hope that this questionnaire does not simply give further evidence to support the continuation of irrational prescribing". The results of many more trials are needed before evidence based prescribing for neuropathic pain can be achieved. Randomised placebo-controlled trials are needed to demonstrate efficacy, and randomised comparative trials are needed to compare efficacy and side effects. Even more knowledge and research will be needed before we can rationally tailor treatments to subgroups of patients with different types of neuropathic pain. Until then, information such as comes from this questionnaire can be useful as a stimulus to reflect on one's own practice, in particular where there is variation from others.
I gratefully acknowledge the assistance of my colleagues in Wales for piloting the questionnaire, to those who kindly completed the questionnaire and Kerry Hood for her help with the statistics.

|
|
Moderate pain |
Severe pain |
||
|
|
n |
% |
n |
% |
|
Carbamazepine |
17 |
20% |
7 |
8% |
|
Anxiolytics |
4 |
5% |
29 |
34% |
|
Tranquillisers |
2 |
2% |
5 |
6% |
|
Other tricyclic antidepressants |
4 |
5% |
1 |
1% |
|
Other opioids |
6 |
7% |
4 |
5% |
|
Lignocaine |
2 |
2% |
10 |
12% |
|
Mexilitine |
8 |
10% |
- |
- |
|
Nerve blocks |
11 |
13% |
15 |
17% |
|
Acupuncture |
16 |
19% |
8 |
9% |
|
Other complementary methods |
4 |
5% |
2 |
2% |
|
Other methods |
10 |
12% |
5 |
6% |
|
Total |
84 |
|
86 |
|
Anxiolytics = benzodiazepines, midazolam, diazepam, 'anxiolytic'
Tranquilliser = chlorpromazine, methotrimeprazine, 'tranquilliser'
Other opioids = oxycodone, fentanyl, tramadol
Nerve blocks = paravertebral block, psoas block, psoas catheter, lumbo-sacral
plexus block, cordotomy, 'nerve block'